What Makes A Neutral Atom
What is a neutral atom?
What is a neutral cantlet? Before yous understand what a neutral atom is, a expert understanding of cantlet is important. So, what is an cantlet?
Definition of atom:
An cantlet is the smallest particle of an element that can be identified with that element. Still confused?
Don't worry! We will break it down and help yous understand with a concrete example.
A piece of pure golden for example is an element.
![]()
Break this piece of pure gold into billions of smaller pieces until the texture feels like powder of fine golden.
Wait! This is still not pocket-sized enough to call it an cantlet. Each tiny piece of fine gilt is composed of many billions smaller pieces called particles.
As nosotros said in the definition, the smallest amidst these particles is called an atom.
You can still use this extremely pocket-sized piece that yous cannot even run across at this bespeak to identify the element which is in our case gold.
Atoms are too small to be seen with visible light. The reason we cannot see an atom has to do with the wavelength of visible light.
Calorie-free is fabricated upwardly of waves. The wavelength of visible low-cal is about 0.000001 meter. However, the size of an atom is 0.000000001 which is much smaller than 0.000001.
Using the highest magnification available, information technology is possible to see a particle that is larger than the wavelength of visible light.
In 1981, a major quantum came with the invention of the scanning tunneling microscope. You tin now come across individual atom with greater details.
If you studied an atom with this device, you will see that an atom has three subatomic particles chosen electrons, protons, and neutrons.
The exception to this rule is the hydrogen cantlet which simply has 1 proton and i electron.
What is a neutral cantlet and then?
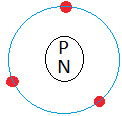
Now that you got the big picture, you lot are set to know what a neutral cantlet is. The picture higher up represents an cantlet.
P = protons
N = neutrons
3 red dots = electrons.
Protons and neutrons form together the nucleus.
The electrons surround the nucleus.
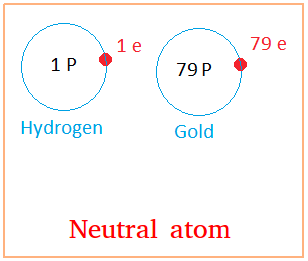
When the number of protons equals the number of electrons, we call the cantlet a neutral atom.
Atoms are therefore neutral in their normal states. Under some circumstances, atoms can lose or gain electrons. In this case, they are no longer neutral. They now have a charge. This lesson about electric charge explicate this in details.
Other examples of neutral atoms.
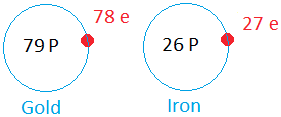
When an cantlet is not neutral, it is called ions. A couple of examples of ions are shown beneath.
Atom quiz
Take the cantlet quiz below to meet how well you understand the cantlet. Later on completing this quiz, you should be able to clearly encounter the difference between a neutral atom and an atom that has an electrical charge.
You lot will non demand to use a paper and pencil to complete this quiz. First, read this lesson nigh neutral cantlet and and then take this quiz.
Objective of the quiz:
- Know what an atom is.
- Know what a nucleus is.
- Know what a neutral atom is
- Know what a particle is
- Know what an element is.
- Know what an ion is.
- Know approximately what the size of an atom is
| Test your knowledge with the quiz below: |
What Makes A Neutral Atom,
Source: https://www.basic-mathematics.com/what-is-a-neutral-atom.html
Posted by: lovelandlosting.blogspot.com


0 Response to "What Makes A Neutral Atom"
Post a Comment